28.05.2025
Studying Shunt Currents in a Water Electrolysis System: A Partnership Between Stargate Hydrogen, ABB and LUT – PART 1
Article Audio summary
If you prefer, you can listen to a summarised version of this article:
Understanding Shunt Currents in Water Electrolysis Systems
Green hydrogen production relies on an efficient water electrolysis system to split water into hydrogen and oxygen. One of the challenges in scaling up this technology is managing electrical losses that can occur due to stray currents, also known as shunt currents. These unwanted currents can reduce overall alkaline system efficiency, leading to higher operational costs.
To address this issue, Stargate Hydrogen, ABB, and LUT University have formed a research partnership to study shunt currents in an alkaline electrolysis elevated-voltage system. By analysing the effects of stray currents in an elevated-voltage alkaline electrolysis setup, the scientists aim to improve efficiency and reduce costs associated with power supply components.
As of present, the study has made steady progress, and the researchers at LUT University were able to measure the individual cell voltages of a smaller setup, comprised of two Stargate special-purpose stacks connected in series.
That is only the starting point of the study that will reach the final stage, where larger systems of up to 10 stacks will be tested while powered by ABB’s power supply.
The Objective of the Study
The primary goal of this study is to reduce system costs and study the connection of alkaline stacks by measuring and analysing stray currents within the balance of plant (BoP) and electrolyser stacks in a water electrolysis system. The research will focus on understanding how shunt currents behave, starting from a 2 stack set-up and moving up until the size of the system reaches the final 10 electrolyser stacks with 48 cells each, identifying the impact of stray currents on current efficiency, and exploring ways to optimise system voltage levels while maintaining stable and efficient operation. To achieve these objectives, researchers will examine how electrolyte hoses connecting the stacks to manifolds influence current leakage.
The cost of power supply systems (Rectifier, transformer, and switchgear) is a significant factor affecting the balance of plant cost and further cost of green hydrogen. Current handling capability is the dominating factor in the cost of power electronics needed for AC to DC rectification. The proportional cost of power electronics can be minimised by increasing the water electrolysis system voltage level towards the 1500 Vdc limit defined by the low voltage directive. Simultaneously, lower current level decreases the cost of other parts of the power supply system, e.g. transformer, busbars, and circuit breakers.
The water electrolysis system voltage level can be increased either by building longer stacks or connecting stacks electrically in series. However, stray currents and mechanical limitations set practical limits for the length of a single stack and finding optimal stack length is an issue for technoeconomic optimisation. Thus, serial connection of relatively short stacks is the chosen approach.
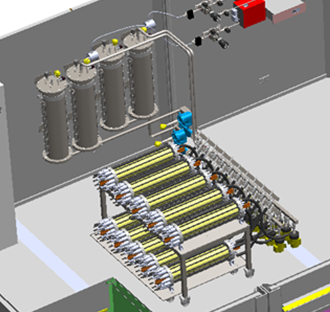
1000 V setup with ten stacks connected in series

Set up with 2 stacks to measure the cell voltage characteristics and the current efficiency of a single stack or two stacks connected in series. The stacks are custom-built by Stargate Hydrogen for the project and are scaled-down versions of industrial alkaline electrolysis stacks.
The Research Setup and Participants
The study aims to be performed, at its conclusion, in a large-scale test system comprising ten electrolyser stacks, each with 48 cells, connected in series. The laboratory-scale stacks, equipped with cell-level voltage probes, are designed and manufactured by Stargate Hydrogen. Key parameters of the test setup include:
- System voltage: 1000 V
- Nominal current: 60 A (selected based on ABB’s industrial power converter, ACS880)
- Grounded steel piping connecting manifolds to separation tanks
- Insulating electrolyte hoses used to connect stacks to manifolds
The participating institutions and key stakeholders involved in the study are:
- Stargate Hydrogen: Rainer Küngas.
- ABB: Simo Säynevirta, Simo Vuorsalo, Matti Kauhanen.
- LUT University: Associate Professor Vesa Ruuskanen, Professor Jero Ahola, Professor Pertti Kauranen, Junior Researcher Toni Viinanen and Markku Niemelä the Research Director.
By combining expertise in electrolyser development, power electronics, and industrial research, the team is well-positioned to generate meaningful results.
The Importance of Industry-Academia Partnerships
Collaboration between stack manufacturers, universities, and engineering companies is essential for advancing technology. These partnerships bring together theoretical knowledge, practical engineering expertise, and industrial scalability, creating a strong foundation for innovation.
By working together on this project, stakeholders can accelerate the development of an efficient and economically viable water electrolysis system. Academic institutions provide the research infrastructure and expertise needed to conduct rigorous experiments, while industry partners offer the manufacturing capabilities and market insight necessary for large-scale implementation.
These collaborations reduce development time, lower costs, and improve the reliability of emerging technologies, making hydrogen production more competitive within the energy market and helping turn scientific discoveries into practical solutions that benefit the entire hydrogen economy.
Advancing Electrolyser Technology
The partnership between Stargate Hydrogen, ABB, and LUT University is just at the beginning and is an important step towards refining water electrolysis system design. The findings from this study will be used to shape future electrolyser development.
Results of the Study
The results will be presented at the ICE2025 conference by Professor Vesa Ruuskanen in August. If you are curious to know more about the results of the full study, keep your eyes open for PART 2.
