11.03.2025
Hydrogen Fuel Cell: NASA’s space technology powering our cars
Main takeaways
- A Hydrogen fuel cell converts chemical energy into electricity through electrochemical reactions between hydrogen and oxygen, producing water and heat as byproducts.
- They are used in transportation, stationary power generation, and portable power applications, offering benefits like high efficiency and zero emissions.
- Compared to gasoline engines and electric motors, hydrogen fuel cells provide higher efficiency and faster refuelling times but face challenges such as hydrogen production and infrastructure development.
- Historically, fuel cells have evolved significantly, with notable milestones like Sir William Grove's first fuel cell in 1839 and Francis Thomas Bacon's development of the hydrogen-oxygen fuel cell in the 1960s.
- Advancements in materials and design have improved fuel cell performance, durability, and cost-effectiveness over the years.
- Looking ahead, hydrogen fuel cells are expected to play a key role in achieving global sustainability goals, particularly in sectors where electrification is challenging.
Introduction
By converting hydrogen into electricity, hydrogen fuel cells offer a clean and efficient power source with water as the only byproduct. Today, hydrogen fuel cells are gaining traction across various industries. Countries around the world are investing heavily in hydrogen infrastructure, and major automotive manufacturers are rolling out fuel-cell vehicles to complement battery-electric options.
Industries such as heavy transport, shipping, and aerospace are exploring fuel cells as viable alternatives to fossil fuels. With governments setting ambitious climate goals, the demand for clean energy solutions is higher than ever, making hydrogen fuel cells a critical piece of the decarbonisation puzzle.
This article explores the fundamentals of hydrogen fuel cells, their applications, historical development, advancements, and prospects.
A brief history of fuel cells
The journey of hydrogen fuel cells spans nearly two centuries, marked by amazing discoveries and technological advancements.
Key milestones in fuel cell development:
- 1839: Sir William Grove developed the first fuel cell prototype with galvanic cells (which laid the groundwork for modern fuel cells)
- 1889: Ludwig Mond and Carl Langer improved Grove’s design, coining the term "fuel cell."
- 1932: Francis Thomas Bacon built the first practical hydrogen-oxygen fuel cell.
- 1960s: NASA used fuel cells to power the Apollo spacecraft.
- 1990s-Present: Advancements in materials and efficiency enabled commercialization in transportation and power generation.
Main improvements in fuel cell designs and performance over the years
Notable advancements:
- Material Enhancements: Use of platinum alternatives to reduce costs.
- Durability Improvements: Fuel cell lifespans have increased, making them viable for commercial use.
- Efficiency Gains: Better catalysts and membrane designs have enhanced energy conversion rates.
- Cost Reductions: Manufacturing processes have become more cost-effective, making fuel cells more accessible.
What are fuel cells and how do they work?
A hydrogen fuel cell is an electrochemical device that combines hydrogen and oxygen to produce electricity, water, and heat. Unlike conventional combustion engines, fuel cells generate power without burning fuel, resulting in zero harmful emissions.
Key components of a hydrogen fuel cell:
- Anode: Where hydrogen gas is supplied and split into protons and electrons.
- Cathode: Where oxygen gas combines with electrons and protons to form water.
- Electrolyte: A medium that allows only ions (for example protons) to pass through, forcing electrons to travel through an external circuit, generating electricity.
Chemical reactions in a hydrogen fuel cell:
The reactions for proton exchange membrane fuel cell, the most common type of fuel cell, are as follows:
- Anode Reaction: H₂ → 2H⁺ + 2e⁻
- Cathode Reaction: ½O₂ + 2H⁺ + 2e⁻ → H₂O
- Overall Reaction: H₂ + ½O₂ → H₂O + Electricity + Heat
This process efficiently converts the chemical energy of hydrogen into electrical energy, with water and heat as the only byproducts.
Types of fuel cell technologies
Not all hydrogen fuel cells operate in the same way. Several types of fuel cells exist, each designed for specific applications, efficiency levels, and operating conditions. Here are the most prominent types:
1. Proton exchange membrane fuel cells (PEMFCs)
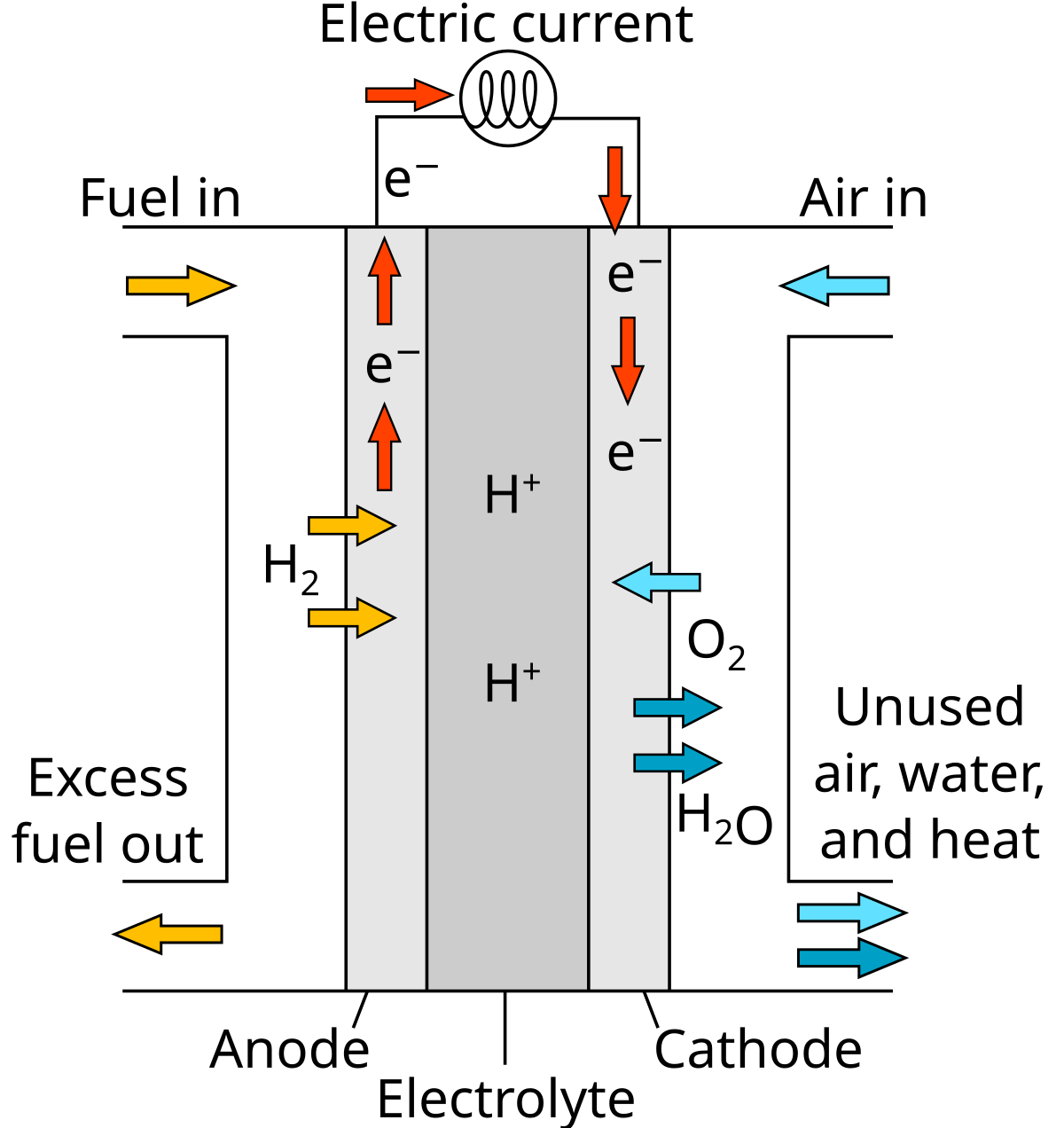
- Operating Temperature: ~80°C
- Efficiency: 50-60%
- Applications: Transportation (cars, buses, trucks), portable power, and backup power.
- Key Features: Lightweight, compact, and capable of rapid startup.
Note that the efficiency range given for Proton Exchange Membrane Fuel Cells (PEMFCs) is 50-60%, but this can vary more depending on the application and operating conditions. In practice, fuel cell efficiencies can reach up to 70-80% when used in Combined Heat and Power (CHP) applications.
2. Solid oxide fuel cells (SOFCs)
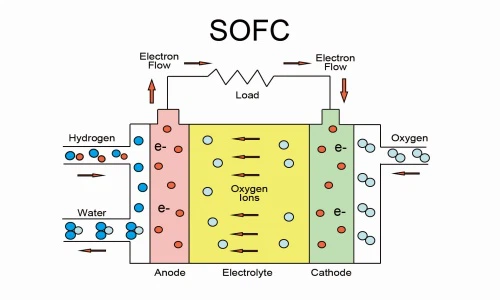
- Operating Temperature: 600-1,000°C
- Efficiency: 60-65% (can reach 85% in combined heat and power systems).
- Applications: Stationary power generation for industrial and commercial use.
- Key Features: High efficiency, tolerance for multiple fuel types (including natural gas and biogas), and long lifespan, but requires longer startup times.
3. Alkaline fuel cells (AFCs)
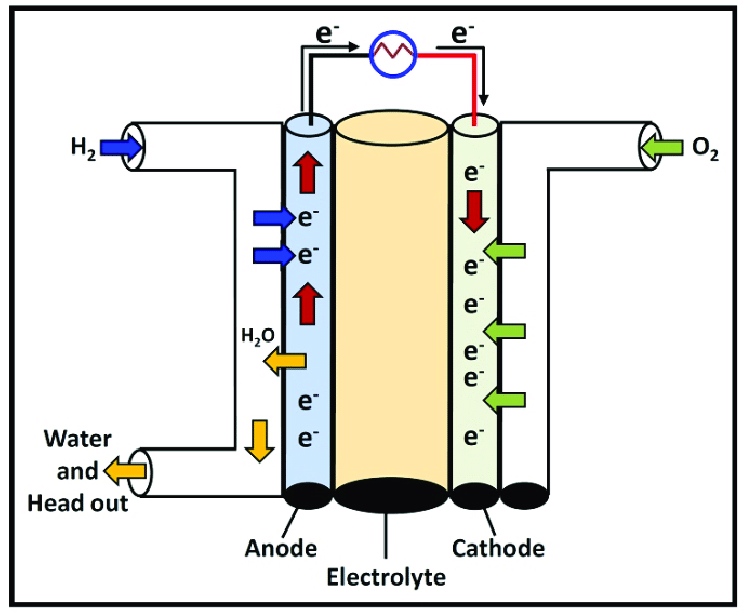
- Operating Temperature: 60-90°C
- Efficiency: 60-70%
- Applications: Space applications (used in Apollo and Space Shuttle programs), potential military and remote power applications.
- Key Features: High efficiency and fast start-up times but sensitive to CO₂ contamination, requiring pure oxygen or air filtration.
4. Phosphoric acid fuel cells (PAFCs)
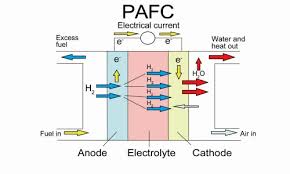
- Operating Temperature: ~200°C
- Efficiency: 40-50%
- Applications: Stationary power generation for commercial buildings and industrial facilities.
- Key Features: Reliable for long-term use, very tolerant to fuel impurities and can employ multiple fuel types, but larger and heavier compared to PEMFCs.
5. Molten carbonate fuel cells (MCFCs)
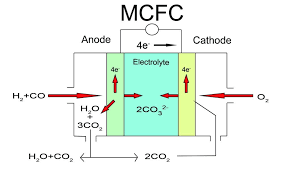
- Operating Temperature: ~650°C
- Efficiency: 50-60% (can exceed 80% in cogeneration).
- Applications: Large-scale power plants, marine applications, and industrial use.
- Key Features: High efficiency and ability to use a variety of fuels, including natural gas and biogas, but requires high operating temperatures and long startup times.
Each of these fuel cell types has its advantages and trade-offs, making them suitable for different industries and use cases. The choice of technology depends on factors like efficiency, cost, operating conditions, and scalability requirements.
The uses of hydrogen fuel cells
The versatility of hydrogen fuel cells allows them to be deployed across multiple industries. Their unique combination of efficiency, scalability, and zero emissions makes them particularly useful in applications where battery-electric technology has limitations.
Transportation:
- Light-Duty Vehicles: Fuel cell vehicles (FCVs) like the Toyota Mirai offer longer driving ranges and shorter refuelling times compared to battery electric vehicles (BEVs).
- Heavy-Duty Vehicles: Fuel cells are ideal for buses, trucks, and trains, providing the necessary power and range without lengthy charging times. In many heavy-duty cases, using batteries is nearly impossible due to the weight of the batteries increasing the necessary power and energy capacity, which in turn increases the required battery pack size and weight. Hydrogen, on the other hand, is very energy-dense.
Stationary Power Generation:
- Backup Power: Fuel cells provide reliable backup power for critical infrastructure, such as hospitals and data centers.
- Distributed Generation: They enable on-site power generation for residential and commercial buildings, reducing transmission losses and enhancing energy security.
Portable Power:
- Electronics: Fuel cells can power portable devices like laptops and cameras, offering longer operational times and lower weight compared to traditional batteries.
- Remote Applications: They are suitable for off-grid locations where conventional power sources are unavailable. Fuel cells have also been seen in military, and emergency backup situations.
Comparison of fuel cells with gasoline engines and electric motors
Understanding the benefits and drawbacks of hydrogen fuel cells requires comparing them to existing technologies, such as gasoline engines and battery-electric motors.
| Key Performance Indicator | Hydrogen Fuel Cells | Gasoline Engines | Electric Motors |
| Efficiency | 50-60% | 25-30% | 85-90% |
| Emissions | water vapor | CO₂, NOₓ, HC | Zero (if re-energy is used) |
| Refueling Time | ~3-5 min | ~5 minutes | 30 minutes to several hours |
| Energy Density | High | High | Low |
| Infrastructure | Limited | Extensive | Growing |
| Maintenance | Low | High | Low |
Here are some important comments about the table above:
Energy Density - The energy density of hydrogen is marked as "High" compared to electric motors, but it’s important to specify that hydrogen has a high gravimetric energy density (energy per unit mass) but a low volumetric energy density (energy per unit volume) compared to conventional fuels, which may affect storage and transportation.
Maintenance: PEM fuel cells (especially when operating on fuel with impurities) can face degradation issues, particularly with platinum catalyst degradation, which affects performance over time. However, since most of the produced hydrogen is purified, degradation due to impure fuel is rare, and only natural long-term degradation may occur.
What is the role of fuel cells in the hydrogen economy?
As nations transition toward clean energy, hydrogen fuel cells will become an important tool for powering the hydrogen economy.
- Decarbonising Transportation: Offering a clean alternative to fossil fuels, especially in sectors where battery electrification is challenging.
- Integrating Renewable Energy: Excess renewable energy can be used to produce hydrogen via electrolysis, which can later be converted back to electricity using fuel cells.
- Enhancing Energy Security: Hydrogen can be produced from various domestic resources, reducing dependence on imported fuels.
But there are challenges to be solved before Hydrogen and Fuel Cells can have a broader impact on our economy, such as:
Challenges of Hydrogen Storage and Distribution
One of the key challenges in the widespread adoption of hydrogen fuel cells is the storage and distribution of hydrogen. Hydrogen is a very light gas and has a low volumetric energy density in its natural state, which presents logistical and engineering difficulties.
To store hydrogen efficiently, it must be either compressed at high pressures (typically 350-700 bar), liquefied at extremely low temperatures (cryogenic storage), or stored in solid form using advanced materials like metal hydrides or chemical carriers.
To know more about hydrogen storage, read our post about the topic HERE>
Global Policy and Market Trends
The global policy landscape plays its role in the future of hydrogen fuel cells. Many governments worldwide are setting ambitious climate goals and making investments to accelerate the adoption of clean energy solutions.
For instance, the European Union and several Asian countries have been actively supporting the development of hydrogen infrastructure through subsidies, tax incentives, and regulations promoting green hydrogen production.
While hydrogen is a highly flammable gas, significant advancements in safety standards, engineering, and technology have made its use in fuel cells safe and reliable. Modern hydrogen storage systems are designed to withstand high pressures and are equipped with multiple safety features, including pressure relief valves, leak detection systems, and automatic shutdown mechanisms.
Safety Aspects
While hydrogen is a highly flammable gas, significant advancements in safety standards, engineering, and technology have made its use in fuel cells safe and reliable. Modern hydrogen storage systems are designed to withstand high pressures and are equipped with multiple safety features, including pressure relief valves, leak detection systems, and automatic shutdown mechanisms.
To know more about hydrogen storage, read our post about the topic HERE>
Public Perception and Acceptance
Public perception of hydrogen fuel cells can be a significant barrier to their adoption. Despite their environmental benefits, hydrogen fuel cells are often met with scepticism due to concerns about safety, fuel production, and the perceived complexity of the technology. Educating the public on the safety features of hydrogen systems, the environmental benefits of green hydrogen, and the advantages of fuel cell technology is crucial.
What is the future of fuel cells?
The future of hydrogen fuel cells looks promising, with key trends including:
- Expansion in Heavy-Duty Transport: Adoption in trucking, shipping, and aviation.
- Increased Infrastructure Development: Growth of hydrogen refueling networks worldwide.
- Policy Support and Investments: Companies and governments are investing in hydrogen fuel cell technology to meet climate goals.
- Breakthroughs in Hydrogen Production: Innovations in green hydrogen production will further drive adoption.
Conclusion
Hydrogen fuel cells have evolved from experimental prototypes to commercially practical energy solutions with applications in transportation, power generation, and industry.
Their ability to provide clean, efficient, and scalable power makes them an important technology in the global transition toward sustainable energy. While challenges such as infrastructure development and hydrogen production remain, ongoing advancements in technology and policy support are increasing their adoption.
Want to learn more about hydrogen Visit Stargate Hydrogen for expert insights and industry-leading solutions.
