11.08.2025
Connecting Lab and Industry to Develop the next generation of Advanced Electrode Materials – by MSc Alise-Valentine Prits
Article Key Takeaways
- Advanced electrode materials, such as Stargate Hydrogen’s ‘Stardust’ material, are the key to improving the efficiency of alkaline water electrolysers (AWE).
- Applying advanced electrode materials in the industry requires laboratory measurements at industrially relevant conditions.
- Key parameters influencing the measurement results of AWE setups include temperature, pressure, electrolyte concentration, and iron content.
- The choice of measurement setup for characterising AWE electrodes should depend on the specific focus of the study.
- Well-designed lab tests can predict the performance of an electrolyser stack.
About the Author

Alise-Valentine Prits is a PhD researcher in sustainable energetics at the University of Tartu and a Junior Scientist at Stargate Hydrogen. Her work focuses on developing the next generation of advanced electrode materials for alkaline water electrolysis, contributing to the advancement of green hydrogen technologies. With a strong foundation in chemistry and materials engineering, Alise-Valentine combines academic insight with hands-on experience in R&D. She is also a teacher and is passionate about making science accessible to everyone.
Introduction
This article aims to provide a practical overview of the findings from the study "Electrochemical characterisation of Raney nickel electrodes for alkaline water electrolysis: From laboratory to industrial scale" by Alise-Valentine Prits, et. al. Published with unrestricted access in the International Journal of Hydrogen Energy.
While it might seem easy and straightforward at first, studying advanced electrode materials for alkaline water electrolysis can be challenging. One example is a recent round-robin test, where 11 laboratories used an identical flow cell setup to evaluate AWE performance. Despite following a carefully developed protocol and using identical electrodes and separators, the results varied by as much as 610 mV at 1000 mA/cm². This highlights how difficult it can be to achieve consistent and repeatable results, even under controlled conditions. Moreover, it is uncertain how the results obtained from laboratory-scale setups correlate with those from larger, scaled-up systems.
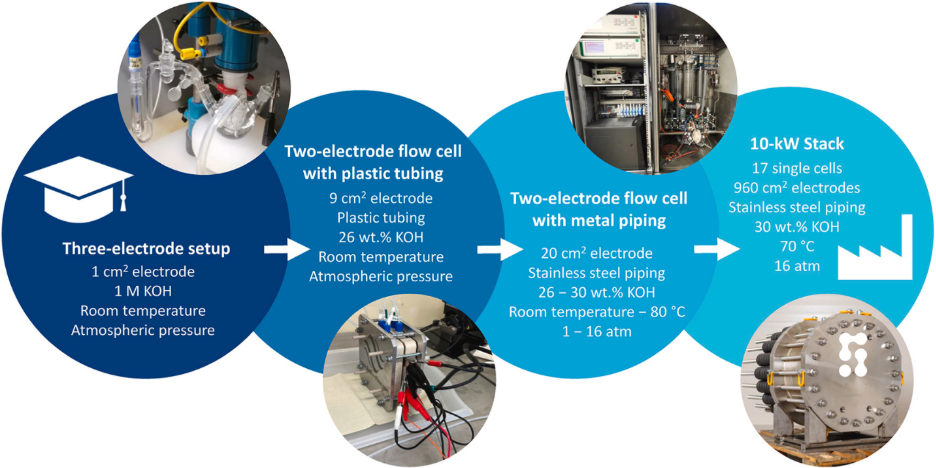
To better understand these problems, Prits, et. al., studied a commercial Raney Ni electrode across a range of electrochemical setups – from a typical three-electrode lab cell to a 10 kW industrial stack. The study explores how different testing parameters affect performance and offers recommendations for improving the transferability of lab-scale research to industrial applications.
Our goal with this article is to translate those insights into a clear and practical perspective, helping companies and researchers design laboratory experiments that yield results more relevant to real-world hydrogen production. Click here if you want to read the study.
Why is it important to study advanced electrode materials?
Electrode materials play a central role in the efficiency and cost of alkaline water electrolysis (AWE). They directly influence how much energy is needed to split water into hydrogen and oxygen by lowering the activation energy of the reactions. The more effective the catalyst, the less energy is wasted, and the more viable green hydrogen becomes.
While noble metals like platinum, iridium, and ruthenium offer excellent performance, their high cost and limited availability make them impractical for large-scale AWE systems. That’s why most commercial electrolysers rely on more affordable nickel-based electrodes, even though they come with a trade-off in efficiency.
To bridge this gap, researchers are actively developing nickel-based advanced electrode materials that offer better performance without relying on scarce elements. Raney nickel is one such example already used in industry, but there is still room for improvement.
Aiming to drastically reduce the cost of green hydrogen production, Stargate Hydrogen’s scientists are developing novel advanced electrode materials, which they call Stardust. A completely new class of electrolysers: ceramics-based alkaline electrolysers, that have high current densities, and high efficiencies, yet contain no precious metals. This results in significantly lower hydrogen production costs and makes the electrolysers affordable for the heavy industries which are one of the largest emitters of CO2 in the atmosphere.
What makes studying advanced electrode materials challenging?
One of the main challenges in developing new advanced electrode materials is that laboratory testing conditions often differ significantly from those used in industrial alkaline water electrolysis (AWE) systems. As a result, findings from academic research may not always translate directly to real-world applications.
In many academic studies, electrodes are tested using three-electrode setups with small electrode areas, no separator, and no electrolyte circulation. These experiments are typically conducted at room temperature and atmospheric pressure, using diluted KOH solutions (e.g., 0.1 M or 1 M), and often benchmarked at low current densities around 10 mA/cm².
In contrast, industrial AWE systems operate under much harsher conditions: concentrated KOH (~30%wt), elevated temperatures (around 80 °C), and pressures up to 30 bar. Modern stacks also run at much higher current densities – up to 1 A/cm². These differences are especially important in durability studies, where current density and temperature can strongly influence electrode lifetime and performance.
Another factor is electrolyte purity. In lab settings, KOH is often purified to remove impurities like iron, which can affect catalytic activity. However, in industrial systems, iron is commonly present due to leaching from stainless steel components. This means that testing materials in iron-free conditions may not reflect how they will behave in actual electrolysers.
Together, these differences highlight why it is essential to design lab experiments that better reflect industrial conditions, especially when the goal is to evaluate materials for real-world use.
What parameters matter the most when studying advanced electrode materials?
To generate results that are relevant for industrial applications, several key factors should be carefully controlled and reported during lab-scale testing:
Temperature
Temperature has a major impact on performance. In this study, increasing the operating temperature from room temperature to 80 °C reduced cell voltage by up to 240 mV at 300 mA/cm². This is mainly due to improved electrolyte conductivity. However, since catalyst behaviour can vary with temperature, testing at elevated temperatures is essential for industrial relevance.
Pressure
Increasing pressure from 1 to 6 atm reduced the voltage by about 40 mV at 300 mA/cm² in this study. While this shows a measurable effect, the impact of pressure was smaller compared to the effect of temperature. This observation is consistent with previous research, which suggests that higher pressure can reduce gas bubble size and minimise bubble blockage, thereby lowering resistance.
Electrolyte composition
In this study, using a more concentrated KOH solution (26% vs. 1 M) led to lower voltages for the oxygen evolution reaction (OER) and the full electrolysis cell. This improvement is mainly due to increased ionic conductivity. For the hydrogen evolution reaction (HER), the effect of KOH concentration was minimal.

The presence of iron in the electrolyte also had a clear activating effect – this was observed in both the three-electrode and two-electrode setups. Adding a small amount of iron (0.1 mM Fe³⁺) reduced OER overpotentials by up to 80 mV and improved full-cell performance, especially at higher current densities. This enhancement is attributed to the formation of catalytically active nickel-iron surface species for OER, and the formation of Fe dendrites leading to increased surface area or the prevention of the formation of deactivating Ni hydride for HER.
Because iron can significantly influence performance, it is important to monitor and control its concentration during testing. This is particularly relevant when the goal is to evaluate advanced electrode materials under conditions that reflect real industrial use, because industrial electrolysers typically include stainless steel components from which iron can leach into the electrolyte over time.
Cell configuration
The study demonstrates that cell design can influence the results. The setup that mimicked the industrial stack more closely by including elastic elements showed higher voltages than the cell without the elastic elements. Yet the performance of this cell was also closer to the performance of the stack. This difference was linked to increased ohmic resistance and variations in flow dynamics. It’s therefore important to define the goal of the measurement and choose the cell configuration accordingly.
Recommendations from the authors
To ensure industrial relevance, the authors recommend performing measurements at temperatures above 70 °C using concentrated KOH solutions (~7 M). The iron content in the electrolyte should be recorded and, where possible, controlled. If the setup allows, testing at pressures above 5 atm is also advised. These conditions reflect the real-world operation of alkaline electrolysers and align with EU testing protocols.
What measurement setups can be used to study advanced electrode materials?

To evaluate advanced electrode materials for alkaline water electrolysis, researchers can choose from a variety of electrochemical setups. These setups differ in complexity, scale, and how closely they reflect the configuration of the cells in actual electrolysers.
In the study discussed here, four types of setups were used to evaluate Raney nickel electrodes. To make the comparison easier to follow, we refer to them using simplified names. The corresponding setup codes from the original article are also included for reference:
- Basic lab cell (3E) – a three-electrode system commonly used in academic research
- Simple flow cell (2EP) – a two-electrode system with plastic tubing and circulating electrolyte
- Advanced flow cell (2EMC1 and 2EMC2) – a two-electrode system with metal piping, capable of operating at higher temperatures and pressures
- Industrial stack (S) – a full-scale 17-cell electrolysis stack used for performance validation
How to choose the setup?
The choice of measurement setup for characterising AWE electrodes should depend on the specific focus of the study. For example, while all four setups can be used to study advanced electrode materials, they serve different purposes depending on the research question, available resources, and desired level of industrial relevance.
Basic Lab Cell
The three-electrode system is ideal for fundamental research. It allows separate analysis of the oxygen and hydrogen evolution reactions (OER and HER), making it especially useful for understanding reaction mechanisms and evaluating catalyst activity. However, it does not include electrolyte circulation or pressure control, and measurements are typically done at room temperature with diluted electrolytes. While it's excellent for early-stage screening, it may not reflect industrial performance.
Simple Flow Cell
This two-electrode setup with plastic tubing introduces electrolyte circulation and a diaphragm, making it more representative of real electrolysis cells. It allows for controlled iron content in the electrolyte and is relatively easy to assemble and modify. The flexibility of using different gaskets makes it suitable for testing various electrode and separator thicknesses. However, this flexibility can also introduce variability between experiments. Although the setup used in this study did not support elevated temperatures, it could be modified to do so. Among all setups, the simple flow cell is the most practical for long-term testing and is recommended for initial screening of advanced electrode materials.
Advanced Flow Cell
This metal-piped two-electrode system is designed for testing under industrially relevant conditions, including elevated temperatures and pressures. In this study, two configurations of the setup were used:
- 2EMC1: A simpler design without elastic elements, easier to assemble
- 2EMC2: A more complex configuration that includes elastic elements and expanded metal sheets, closely mimicking the design of the industrial stack
The fixed geometry of the anode and cathode compartments helps ensure consistent assembly between experiments. The system also supports pressurised operation and can be equipped with gas analysers to study gas purity and crossover. However, it is more complex and time-consuming to use, and the electrolyte composition cannot be precisely controlled due to iron and chromium leaching from stainless steel components. This setup is best suited for secondary screening of promising materials and for estimating stack-level performance without the need for full-scale testing.
Industrial Stack

The full-scale 17-cell stack is used to validate electrode performance under real-world operating conditions. While it provides the most accurate performance data, it requires significantly more resources and is not practical for early-stage testing. Stack testing is essential for evaluating system-level effects such as shunt currents, which cannot be replicated in single-cell setups.
Quick Guide to Setup Selection
| Basic lab cell | Best for mechanistic studies and early-stage catalyst development. |
| Simple flow cell | Ideal for initial screening and long-term testing, especially when precise control over electrolyte composition is important. |
| Advanced flow cell | Suitable for testing under industrial-like conditions and for secondary screening of promising materials. |
| Industrial stack | Well-suited for final validation and for studying effects that cannot be captured in single-cell setups. |
How do the results measured with laboratory-scale setups correlate with industrial-scale setups?
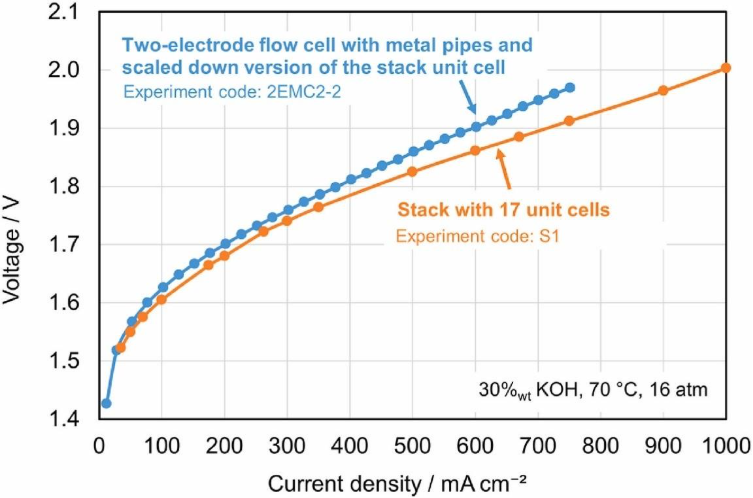
The study demonstrates that results from laboratory-scale setups can closely reflect the performance of full-scale electrolysis systems when the lab configuration is appropriately designed.
The advanced flow cell, which incorporates metal piping and elastic elements, showed a strong alignment with the industrial stack under identical operating conditions (30% KOH, 70 °C, 16 atm). The voltage difference between the two setups was less than 20 mV at low current densities and around 60 mV at higher current densities.

In comparison, the other configuration of the advanced flow cell, which did not include the elastic element, exhibited greater deviation from the industrial stack. This suggests that, in addition to industrially relevant measurement conditions, replicating mechanical and flow characteristics is important for improving the accuracy of lab-scale predictions.
While the advanced flow cell provides a practical and resource-efficient method for estimating industrial performance, full-stack testing remains necessary for evaluating system-level phenomena such as gas crossover and shunt currents.
Conclusion
Bridging the gap between lab-scale experimentation and industrial performance is no longer a theoretical challenge; it is a practical necessity. Developing advanced electrode materials is the key to more efficient electrolysers, but testing them under the right conditions is what turns academic results into scalable industrial solutions. As demonstrated in the research by Alise-Valentine Prits and team, each test setup, from three-electrode systems to pressurised two-electrode cells, has a role to play in a structured R&D program.
By applying the right tools in the right order and paying attention to conditions like Fe content, temperature, and KOH concentration, researchers and engineers can build a dependable bridge between lab results and megawatt-scale hydrogen production.
By investing in novel catalysts like Stargate Hydrogen’s Stardust and refining testing protocols to reflect real-world environments, the industry moves closer to delivering scalable, affordable, and high-efficiency alkaline electrolysers. In doing so, we not only improve technological performance but also take a critical step toward decarbonising heavy industry and accelerating the global energy transition.
Let’s Work Together
At Stargate Hydrogen, we take pride in bringing technical expertise and industrial realism to every electrolysis project. If you’re looking to kickstart hydrogen production, get in touch with us today. We’re here to help turn proven lab research into real-world hydrogen output.
